by Adarsh S.M(2016-2019)
https://www.facebook.com/adarsh.asm.7
Thermodynamics, Science of the relationship between heat ,work,temperature and energy. In broad terms, thermodynamics deals with the transfer of energy from one place to another and from one form to another. The key concept is that heat is a form of energy corresponding to a definite amount of mechanical work.

Heat was not formally recognized as a form of energy until about 1798, when Count Rumford (Sir Benjamin Thomson), a British military engineer, noticed that limitless amounts of heat could be generated in the boring of cannon barrels and that the amount of heat generated is proportional to the work done in turning a blunt boring tool. Rumford’s observation of the proportionality between heat generated and work done lies at the foundation of thermodynamics. Another pioneer was the French military engineer Sadi Carnot, who introduced the concept of the heat-engine cycle and the principle of reversibility in 1824. Carnot’s work concerned the limitations on the maximum amount of work that can be obtained from a steam engine operating with a high-temperature heat transfer as its driving force. Later that century, these ideas were developed by Rudolf Clausius, a German mathematician and physicist, into the first and second laws of thermodynamics, respectively.
Statistical mechanics

Statistical mechanics is a branch of theoretical physics that uses probability theory to study the average behaviour of a mechanical system whose exact state is uncertain.[1][2][3][note 1]
Statistical mechanics is commonly used to explain the thermodynamic behaviour of large systems. This branch of statistical mechanics, which treats and extends classical thermodynamics, is known as statistical thermodynamics or equilibrium statistical mechanics. Microscopic mechanical laws do not contain concepts such as temperature, heat, or entropy; however, statistical mechanics shows how these concepts arise from the natural uncertainty about the state of a system when that system is prepared in practice. The benefit of using statistical mechanics is that it provides exact methods to connect thermodynamic quantities (such as heat capacity) to microscopic behaviour, whereas, in classical thermodynamics, the only available option would be to just measure and tabulate such quantities for various materials. Statistical mechanics also makes it possible to extend the laws of thermodynamics to cases which are not considered in classical thermodynamics, such as microscopic systems and other mechanical systems with few degrees of freedom.
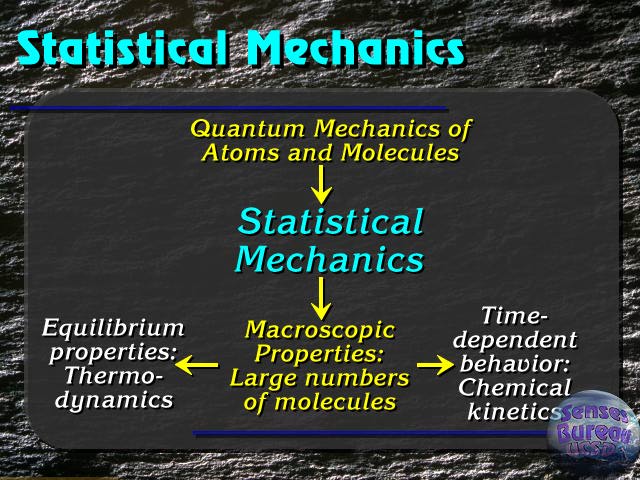
Statistical mechanics also finds use outside equilibrium. An important subbranch known as non-equilibrium statistical mechanics deals with the issue of microscopically modelling the speed of irreversible processes that are driven by imbalances. Examples of such processes include chemical reactions or flows of particles and heat. Unlike with equilibrium, there is no exact formalism that applies to non-equilibrium statistical mechanics in general, and so this branch of statistical mechanics remains an active area of theoretical research.
